Altana and Wyeth were thwarted in their attempt to block Teva Pharmaceuticals from infringing U.S. Patent No. 4,758,579, directed to the compound pantoprazole, the active ingredient in Altana’s antiulcer drug Protonix®. The compound pantoprazole belongs to a class of compounds known as proton pump inhibitors (“PPIs”) that are used to treat gastric acid disorders in the stomach.
Although the operation of the gastric acid pump was known at the time of the invention at issue, the mechanism by which PPIs inhibit the gastric acid pump was not understood in the art until after the effective filing date of the ’579 patent. Part of the uncertainty surrounding the method of action for PPIs is attributable to the fact that PPIs are prodrugs, which are drugs that convert to their active form after they are delivered within a patient’s body, which typically exhibits a pH of about 5 to about 7. In this regard, PPIs are acid-activated prodrugs that are converted into their active form in the highly acidic environment, having a pH of about 1, within the secretory canaliculus of parietal cells. Once converted to its active form, the PPI thereafter binds to one or more cysteine amino acids in the acid pump. This binding inhibits the operation of the gastric acid pump.
Altana’s research efforts resulted in the issuance of U.S. Patent No. 4,555,518 (“the ’518 patent”) and the ’579 patent. The application for the ’518 patent was filed before the ’579 patent, and contained a pharmacology section that compared the effectiveness of 18 claimed compounds against four prior art compounds. The ‘518 patent refers to one of the 18 compounds chosen for testing as compound 12. The ’579 patent, which is not related to the ’518 patent, claims PPI compounds that are structurally similar to the compounds claimed in the ’518 patent. Pantoprazole, the compound at issue in this litigation, exhibits a structure that is very similar to compound 12 from the ’518 patent.
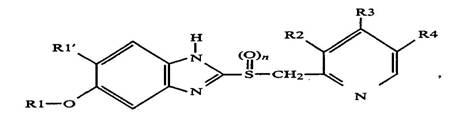
There are three main structural elements to the PPI molecular backbone: the benzimidazole ring, the methylsulfinyl bridge, and the pyridine ring. The general formula of the PPI disclosed in the ’579 patent is reproduced below:
The issues in this case primarily relate to the pyridine ring (the right-most structure on the above compound), specifically, the radicals located on the pyridine ring (indicated by R2, R3, and R4). The ’579 patent teaches that “R3 represents a 1-3C-alkoxy radical, one of the radicals R2 and R4 represents a 1-3C-alkoxy radical and the other represents a hydrogen atom (–H) or a 1-3C-alkyl radical.”
Teva filed an Abbreviated New Drug Application (“ANDA”) requesting FDA approval to sell a generic version of Protonix® prior to the expiration of the ’579 patent along with paragraph IV certifications. Altana filed a motion for preliminary injunction and Teva and Sun conceded infringement; however, they maintained that the ’579 patent is invalid. Specifically, they argued that the ’579 patent was obvious in light of the teachings in Altana’s ’518 patent, the Sachs article, the Bryson article, and the ’431 patent (covering omeprazole).
The obviousness analysis focused on the selection of compound 12 from the ’518 patent as a lead compound for modification. The defendants argued that the Sachs article provided motivation for one of skill in the art to lower the pKa of a PPI to a value of 4 in order to provide better stability of the compound in the patient’s body.
The district court found that the defendants had demonstrated a substantial question of invalidity and the plaintiffs had not shown that it lacked substantial merit. In particular, the court found that one of skill in the art would have selected compound 12 as a lead compound for modification.
So, the district court denied the motion for preliminary injunction. Altana Pharma AG and Wyeth went crying to the Court of Appeals for the Federal Circuit and the Federal Circuit said “too bad.” Altana Pharma Ag v. Teva Pharma (08-1039).
To obtain a preliminary injunction, a court examines four factors:
- a reasonable likelihood of success on the merits;
- irreparable harm if an injunction is not granted;
- a balance of hardships tipping in its favor; and
- the injunction’s favorable impact on the public interest.
Altana argued that the district court was wrong for allowing the defendants to select compound 12 of the ’518 patent as a lead compound when the prior art suggested the availability of numerous other compounds that were at least as promising to modify as compound 12.
This Federal Circuit has said that “[w]here, as here, the patent at issue claims a chemical compound, the analysis of the third Graham factor (the differences between the claimed invention and the prior art) often turns on the structural similarities and differences between the claimed compound and the prior art.” Eisai Co. Ltd. v. Dr. Reddy’s Labs., Ltd., 533 F.3d 1353, 1356-57 (Fed. Cir. 2008). Thus, to establish a prima facie case of obviousness in cases involving new chemical compounds, the accused infringer must identify some reason that would have led a chemist to modify a known compound in a particular manner.
Obviousness based on structural similarity may be proven by the identification of some motivation that would have led one of ordinary skill in the art to select and modify a known compound in a particular way to achieve the claimed compound. The requisite motivation can come from any number of sources and need not necessarily be explicit in the art. Instead, “it is sufficient to show that the claimed and prior art compounds possess a ‘sufficiently close relationship . . . to create an expectation,’ in light of the totality of the prior art, that the new compound will have ‘similar properties’ to the old.”
In the end, the Federal Circuit agreed with the district court’s finding that the defendants raised a substantial argument that compound 12 was a natural choice for further development in this regard:
Ample evidence supported this finding. First, the ’518 patent claimed that its compounds, including compound 12, were improvements over the prior art, specifically omeprazole (the first successful PPI). In addition, compound 12 was disclosed as one of the more potent of the eighteen compounds of the ’518 patent for which data was provided during prosecution. Moreover, the patent examiner relied on the compounds of the ’518 patent during the prosecution of the ’579 patent. Cf. Eisai, 533 F.3d at 1357 (“Indeed, Teva’s pharmacology expert . . . declined to opine on lansoprazole’s relevance to an examiner assessing the patentability of rabeprazole.”).
Beyond the finding that those of skill in the art would have pursued the 18 exemplary compounds in the ’518 patent, the district court also found that one of skill in the art would have found compound 12, in particular, a natural choice for further development efforts. This finding is supported by evidence that compound 12 was one of the more potent PPI compounds disclosed in the ’518 patent. Although potency is not dispositive, the district court believed–not unreasonably–that the potency of the compound was a factor that would have led one of skill in the art to select compound 12 from the group for further study.