According to the Commission’s complaint, filed in the United States District Court for the Central District of California, Watson and Par, through its partner Paddock Laboratories, each sought regulatory approval from the FDA to market generic versions of AndroGel. In their FDA filings, both companies certified that their products did not infringe the only patent Solvay had relating to AndroGel, and that the patent was invalid. The complaint charges that Solvay agreed to pay the generic companies to abandon their patent challenges and agree not to bring a generic AndroGel product to market for nine years, until 2015.
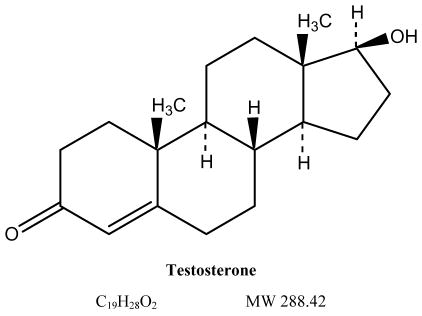
The court action seeks to promote competition between Solvay and generic drug makers that had sought to introduce generic versions of the branded prescription drug AndroGel. AndroGel, Solvay’s second highest selling pharmaceutical product, is a pharmaceutical gel containing synthetic testosterone. It is approved for testosterone replacement therapy in men with low testosterone levels, which often are associated with advancing age, certain cancers, and HIV/AIDS, among other conditions.
The complaint alleges that Solvay acted unlawfully to eliminate generic competition by paying Watson and Par a share of its AndroGel profits to abandon their patent challenges and agree to delay generic entry until 2015 (known as “reverse payments“). As a result, the complaint states that the defendants are cooperating on the sale of AndroGel and sharing the monopoly profits, rather than competing.
According to the Commission’s complaint, defendants’ agreements to eliminate generic AndroGel competition were, and continue to be, unfair methods of competition that violate Section 5(a) of the FTC Act. The FTC says that such payments, also called “exclusion payment settlements,” hinder the intent of the Hatch-Waxman Act of 1984.
The Commission is seeking a final court judgment declaring that Solvay’s agreements with Watson and with Par and Paddock violate Section 5(a) of the FTC Act, and injunctive relief restoring competitive conditions and barring the defendants from engaging in similar or related conduct in the future.
Today, senior Senate Judiciary Committee members Herb Kohl (D-WI) and Chuck Grassley (R-IA) unveiled The Preserve Access to Affordable Generics Act to prohibit brand-name drug manufacturers from using reverse payment agreements to keep generic equivalents off the market.
Sen. Herb Kohl (D-Wis.) had proposed a bill last year along with Sens. Dick Durbin, D.-Ill., and Charles Grassley, R.-Iowa; and now-President Barack Obama.
See the Civil Complaint [Public Version]

[…] mentioned earlier, not only has the Federal Trade Commission has filed a complaint in federal district court […]